Abstract
Introduction:
Depth of response is associated with long-term outcomes in multiple myeloma (MM); however, the effect of response kinetics on outcomes in NDMM is variable and less clear. Although some studies have shown that achieving a ≥very good partial response (VGPR) at 4 months (mos) from diagnosis is associated with increased overall survival (Garderet Leukemia 2018), other reports have shown worse outcomes in pts with early vs late responses (Yan Blood Adv 2019). The double-blind, randomized TOURMALINE-MM2 (NCT01850524) trial (Facon Blood 2021) showed a clinical meaningful progression-free survival (PFS) benefit with IRd vs pbo-Rd (median 35.3 vs 21.8 mos; hazard ratio, 0.830; 95% confidence interval, 0.676-1.018; P=0.073; median follow-up, 53.3 and 55.8 mos, respectively) in NDMM pts. Safety data were consistent with the established toxicity profile of IRd. We evaluated PFS and duration of response (DOR) by depth of best confirmed response and time to best response in TOURMALINE-MM2.
Methods:
Pts were randomized to receive oral ixazomib 4 mg (n=351) or placebo (n=354) on days 1, 8, and 15, plus oral lenalidomide 25 mg (10 mg if creatinine clearance ≤60 mL/min) on days 1-21 and oral dexamethasone 40 mg (20 mg in pts aged >75 years) on days 1, 8, 15, and 22 in 28-day cycles. After 18 cycles, treatment was continued without dexamethasone and reduced doses of ixazomib (3 mg) and lenalidomide (10 mg) until progressive disease (PD)/toxicity. Response assessments were performed every cycle until PD, or every 4 weeks in pts who discontinued treatment prior to PD. PFS and DOR were analyzed post-hoc in subgroups defined by depth of response and in subgroups defined by time to best confirmed response; 'early' and 'late' responses were defined by time to best confirmed response of 0-4 and >4 mos, respectively (additional analyses were performed by time to best confirmed response of 0-6 and >6 mos). Pts in either subgroup could have recorded an initial response prior to their best response. PFS and DOR were evaluated from randomization to progression and time of initial response to progression, respectively. To address potential guarantee-time bias in the PFS analysis, and to eliminate potential bias due to transient responses in DOR analysis, sensitivity analyses were conducted in pts with PFS / DOR of ≥6 mos.
Results:
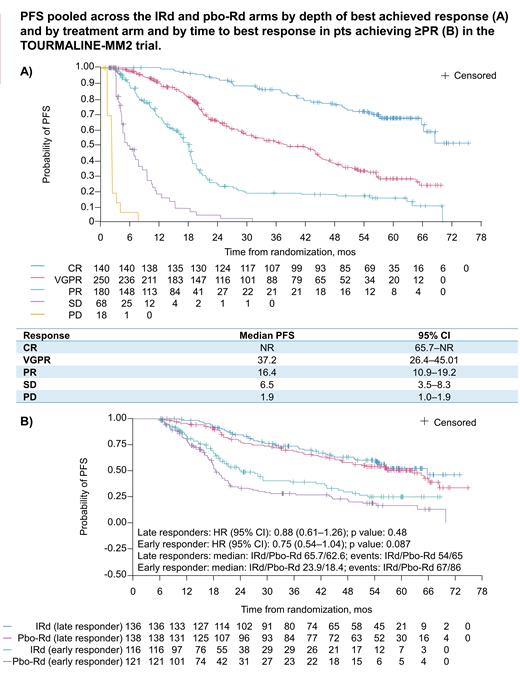
Among the 705 pts in the intention-to-treat (ITT) population, 20% (26% IRd vs 14% placebo-Rd) had a best confirmed response of complete response (CR) or stringent CR, 35% (37 vs 34%) had VGPR, 26% (19 vs 32%) had PR, 10% (9 vs 10%) had stable disease (SD), 3% (1 vs 4%) had PD. 7% (8 vs 6%) of pts were not evaluable. In a pooled analysis of both arms, achieving a deeper response was associated with longer PFS (Figure, A) and DOR (median not reached [NR], 42.8, and 15.0 mos for pts with ≥CR, VGPR, and PR, respectively). In 570 pts with ≥PR (288 IRd; 282 pbo-Rd), 152 (53%) and 136 (47%) in the IRd arm and 143 (51%) and 139 (49%) pts in the pbo-Rd arm were defined as early (0-4 mos) and late (>4 mos) responders, respectively. For early vs late responders, 46% vs 40% were aged ≥75 years, 21% vs 12% had International Staging System stage III MM, and 44% vs 33% had expanded high-risk cytogenetic abnormalities. Median PFS was prolonged among late vs early responders with IRd (65.7 vs 21.2 mos) and pbo-Rd (62.6 vs 18.2 mos), as was median DOR (IRd, NR vs 22.6 mos; pbo-Rd, 64.1 vs 17.2 mos). The PFS sensitivity analysis among pts with PFS of ≥6 mos confirmed the association of late response with improved outcomes; among late vs early responders achieving ≥PR, median PFS was 65.7 vs 23.9 mos with IRd and 62.6 vs 18.4 mos with pbo-Rd (Figure, B), and among late vs early responders achieving ≥VGPR, median PFS was 65.7 vs 35.3 mos with IRd and 64.4 vs 21.7 with pbo-Rd.
Conclusions:
Achieving a deeper response was associated with prolonged PFS and DOR in NDMM patients in TOURMALINE-MM2. PFS benefit on the ITT analysis was driven by the higher rates of deep responses (≥VGPR) with IRd vs pbo-Rd. PFS and DOR were also longer in pts achieving a late vs early best confirmed response of ≥PR or ≥VGPR. Consistent with results from a similar analysis in relapsed/refractory MM in the TOURMALINE-MM1 trial (Garderet Leukemia 2018), our findings support the continuation of therapy with the aim of achieving a deeper response over time. Additional sensitivity analyses will be presented.
Richardson: Sanofi: Consultancy; AstraZeneca: Consultancy; Celgene/BMS: Consultancy, Research Funding; GlaxoSmithKline: Consultancy; Regeneron: Consultancy; Oncopeptides: Consultancy, Research Funding; Protocol Intelligence: Consultancy; Secura Bio: Consultancy; Janssen: Consultancy; Takeda: Consultancy, Research Funding; AbbVie: Consultancy; Karyopharm: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding. Venner: Takeda: Honoraria; BMS: Honoraria; Janssen: Honoraria; Pfizer: Honoraria; Sanofi: Honoraria; GSK: Honoraria. Bahlis: GlaxoSmithKline: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Genentech: Consultancy; Sanofi: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria. White: Amgen: Consultancy, Honoraria; Antengene: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Forus: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria. Karlin: Celgene-BMS: Honoraria, Other: member of advisory board; Sanofi: Honoraria; oncopeptide: Honoraria; Janssen: Honoraria, Other: member of advisory board, travel support; Abbvie: Honoraria; GSK: Honoraria, Other: member of advisory board; Amgen: Honoraria, Other: travel support and advisory board ; Takeda: Honoraria, Other: member of advisory board. Rigaudeau: Takeda: Membership on an entity's Board of Directors or advisory committees. Suzuki: Bristol-Myers Squibb: Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; ONO: Honoraria; Novartis: Honoraria; Sanofi: Honoraria; Abie: Honoraria; Janssen: Consultancy, Honoraria. Shibayama: Mundi Pharma: Honoraria; Otsuka: Honoraria; Pfizer: Honoraria; Bristol-Myers Squibb: Honoraria; Sanofi: Honoraria; Nippon Shinyaku: Honoraria; Fujimoto: Honoraria; Daiichi Sankyo: Honoraria; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Essentia Pharma Japan: Research Funding; Chugai: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Eisai: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Avvie: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Ono: Honoraria, Research Funding; Celgene: Research Funding. Zhang: Takeda: Current Employment. Kumar: Takeda: Current Employment, Current holder of stock options in a privately-held company. Twumasi-Ankrah: Takeda: Current Employment. Labotka: Takeda: Current Employment. Rifkin: Takeda: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Fresenius-Kabi: Membership on an entity's Board of Directors or advisory committees; Coherus: Membership on an entity's Board of Directors or advisory committees; McKesson: Current Employment, Current equity holder in publicly-traded company; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb (Celgene): Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees. Lonial: AMGEN: Consultancy, Honoraria; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Consultancy, Honoraria, Research Funding; GlaxoSmithKline: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Merck: Honoraria. Kumar: Antengene: Consultancy, Honoraria; Carsgen: Research Funding; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Consultancy; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bluebird Bio: Consultancy; Beigene: Consultancy; Tenebio: Research Funding; Roche-Genentech: Consultancy, Research Funding; Novartis: Research Funding; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Research Funding. Moreau: Amgen: Honoraria; Oncopeptides: Honoraria; Janssen: Honoraria; Celgene BMS: Honoraria; Sanofi: Honoraria; Abbvie: Honoraria.
Use of the oral proteasome inhibitor ixazomib in newly diagnosed multiple myeloma patients who are ineligible for transplant.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal